Blog

#bioPGH: Winter Biochemistry - Nature’s Antifreezes
 A resource of Biophilia: Pittsburgh, #bioPGH is a weekly blog and social media series that aims to encourage both children and adults to reconnect with nature and enjoy what each of our distinctive seasons has to offer.
A resource of Biophilia: Pittsburgh, #bioPGH is a weekly blog and social media series that aims to encourage both children and adults to reconnect with nature and enjoy what each of our distinctive seasons has to offer.
It’s been a chilly week in Pittsburgh! While most of us snuggle up in blankets, maybe even by a crackling fireplace, how do plants, animals, even fungi and insects stay alive in freezing weather? Most of our furred and feathered neighbors have insulating layers of, well, fur or feathers to keep warm, but what about the living things with no coats? It turns nature out has a fascinating chemical* plan for winter weather management. So let’s combine a little biology with chemistry and explore!
First things first: the real danger of winter for many creatures is not merely freezing: it is the formation of ice crystals. Ice can damage cells by rupturing them or by damaging valuable compounds the cells use on a regular basis. Different organisms have a variety of ways of preparing for freezing conditions, and they often employ multiple tactics. Trees, for instance, drop their leaves in the winter because the energy that would be needed to repair frozen leaf tissue would be more costly than simply growing new leaves in the spring. Of the insects and arachnids who overwinter, many beat the cold by burrowing underground or some other hiding spot that may remain (hopefully) ice free. Those who are waiting for spring often enter a “hibernation” of sorts known as diapause.
In all of these scenarios, though, organisms’ actions are coupled with natural antifreezes—chemical compounds produced by the organism to either prevent the organism from freezing (freeze-avoiding) or to allow the organism to “safely” freeze (freeze-tolerant). Below are a few of the most common types of natural antifreezes found in our living world. The list is by no means exhaustive, though; part of that is because we simply do not yet know all of the ways that plants and animals can protect themselves from freezing. Are any young biologists up for a challenge?
Sugars—A wide variety of plants, insects, and amphibian species use different combinations of sugars to help prevent freezing. The sugars can lower the freezing point of the fluid in the body, but more importantly, they can help prevent the formation of crystals. One commonly produced sugar alcohol is glycerol, which is used, for example, by the notorious invasive insect emerald ash borer. Many of our native frogs actually produce glucose as an overwintering antifreeze and trees are well-known for their ability to break down their starch reserves to create sugary antifreeze solutions.
Glycolipids—As their name suggests, these compounds contain a sugar component and lipid (fat) component. Most of the antifreeze properties of glycolipids seem to revolve around the cell membranes, though the exact mechanism is not always clear. Until recently, the use of glycolipids as an antifreeze was most commonly associated with insects, but the protective property has been identified in some plants and at least one frog.
Ice-binding proteins—Perhaps more common in polar species, ice-binding proteins are special compounds produced by the cells of some plants, fungi, insects, and even the occasional algae. Ice-binding proteins work by interrupting the formation of ice crystals and by lowering the freezing point, not unlike road salt; but some ice-binding proteins found in plants are orders of magnitude more effective at manipulating the freezing point than anything we humans have used.
Well now, it might seem easier to make a cup of tea and find a cozy blanket, but nature certainly has a way of making sure even the northernmost of beetles have a way to power through a cold winter!
*The word “chemical” has acquired a negative connotation in recent years, but really, everything is made of chemicals. Our bodies’ cells are made of chemicals, water is a chemical, and the air we breathe is composed of chemicals. Granted, there are certainly harmful chemicals! However, the word “chemical” itself refers to neither an innately harmful nor beneficial substance. The word chemical throughout this post is being used to name naturally-occurring compounds.
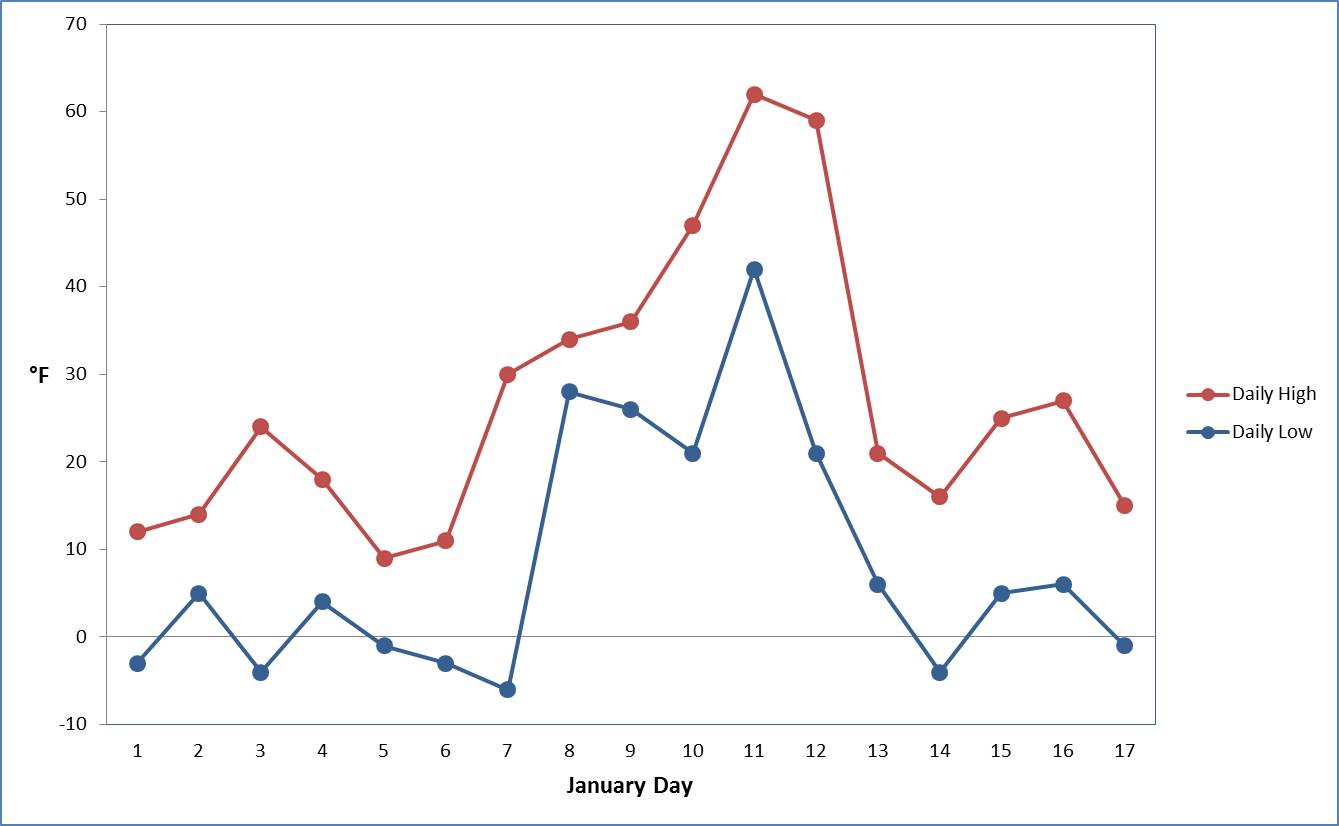
Connecting to the Outdoors: For days that are too cold to play outside, families can explore a little bit of data at home and practice some graphing! To create the graph below, first find your data the National Weather Service’s page for Pittsburgh. From this page, look under the heading “Observed Weather Reports” for the Product “Preliminary Monthly Climate Data,” and hit “Go.” This will open a new window that contains high and low temperatures along with some other data for the month thus far. Then, in an Excel spreadsheet, fill in three columns with the January date, daily high temperature and daily low temperature. Next, select your daily high and low columns (including the column headings!) and while they are highlighted, select the Excel tab “Insert” and select the option for a 2-D Line with Markers Chart. Voila! If you would like to change the color of daily high and low temperatures, right click on data points and select “Format Data Series.” Of course, you may have your own favorite way to make a graph in other programs, or you may be interested in displaying other data instead. Just have fun with data!
Continue the Conversation: Share your nature discoveries with our community by posting to Twitter and Instagram with hashtag #bioPGH, and R.S.V.P. to attend our next Biophilia: Pittsburgh meeting.
Resources
MSU Extension: Winter dormancy and chilling in woody plants
NY Times: When Built-in Antifreeze Beats a Winter Coat
Research Collaboratory for Structural Bioinformatics: Antifreeze Proteins
Sømme 1982: Supercooling and winter survival in terrestrial arthropods
Smithsonian Institute: Where Do Insects Go in the Winter?
Gupta and Deswall 2014: Antifreeze proteins enable plants to survive in freezing conditions

